News
Press Releases
NEW YORK, July 23, 2020 /PRNewswire/ -- BrainStorm Cell Therapeutics Inc. (NASDAQ: BCLI), a leading developer of adult stem cell therapies for neurodegenerative diseases, announced today that it has successfully completed its first milestone in developing an innovative exosome-based platform-technology for the treatment severe COVID-19 infection.
COVID-19 induced pneumonia carries a high fatality rate and has been associated with acute respiratory distress syndrome (ARDS). Currently, there is no effective treatment strategy to prevent or reverse ARDS, a type of respiratory failure associated with widespread inflammation and lung damage mediated by dysregulated cytokine production.
Exosomes are nano-sized (30–150 nm) vesicles secreted by all cell types. Mesenchymal stem cells (MSC) derived exosomes have been suggested as a potential treatment for ARDS due to their ability to penetrate into deep tissues, effectively deliver bioactive molecules to target cells and mitigate the inflammatory response. MSC exosomes may be delivered intravenously or directly into the lung via intratracheal administration.
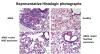
Results from a study in a mouse model of lipopolysaccharide (LPS)-induced ARDS showed that intratracheal administration of NurOwn® (MSC-NTF cells) derived exosomes resulted in a statistically significant improvement in multiple lung parameters. These included the clinically relevant factors: functional lung recovery, reduction in pro-inflammatory cytokines and most importantly, attenuation of lung damage. Moreover, MSC-NTF cell derived exosomes exhibited a superior effect when compared to treatment with exosomes derived from naïve mesenchymal stem cells (MSC) from the same donor. BrainStorm intends to accelerate submission of these important results to peer-reviewed medical journals.
The preclinical experiment demonstrated a statistically significant reduction in lung disease severity score (p=0.03) (based on the American Thoracic Society Documents, 2011 ; Matute-Bello et al., Am J Respir Cell Mol Biol 44;725-738, 2011). and vastly improved lung histology following intratracheal administration of NurOwn (MSC-NTF) exosomes compared to MSC exosomes.
To-date, BrainStorm Cell Therapeutics has focused its clinical development on the application of the NurOwn technology platform to neurodegenerative disorders. Given these important pre-clinical results, the Company will revisit its strategy to determine if and when to proceed with a clinical trial in ARDS.
Dr. Revital Aricha, VP of Research & Development at BrainStorm, will discuss the results on the company’s second quarter 2020 earnings call and corporate update, scheduled for Wednesday August 5th, at 8.00am ET.
About NurOwn®
The NurOwn technology platform (autologous Mesenchymal stem cells, MSC-NTF cells) represent a promising investigational therapeutic approach to targeting disease pathways important in neurodegenerative disorders. MSC-NTF cells are produced from autologous, bone marrow-derived mesenchymal stem cells (MSCs) that have been expanded and differentiated ex vivo. MSCs are converted into MSC-NTF cells by growing them under patented conditions that induce the cells to secrete high levels of neurotrophic factors (NTFs). Autologous MSC-NTF cells can effectively deliver multiple NTFs and immunomodulatory cytokines directly to the site of damage to elicit a desired biological effect and ultimately slow or stabilize disease progression.
About BrainStorm Cell Therapeutics Inc.
BrainStorm Cell Therapeutics Inc. is a leading developer of innovative autologous adult stem cell therapeutics for debilitating neurodegenerative diseases. The Company holds the rights to clinical development and commercialization of the NurOwn technology platform used to produce autologous MSC-NTF cells through an exclusive, worldwide licensing agreement. Autologous MSC-NTF cells have received Orphan Drug status designation from the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of amyotrophic lateral sclerosis (ALS). BrainStorm has fully enrolled a Phase 3 pivotal trial in ALS (NCT03280056), investigating repeat-administration of autologous MSC-NTF cells at six U.S. sites supported by a grant from the California Institute for Regenerative Medicine (CIRM CLIN2-0989). The pivotal study is intended to support a filing for U.S. FDA approval of autologous MSC-NTF cells in ALS. BrainStorm is also conducting a U.S. FDA approved Phase 2 open-label multicenter trial in progressive multiple sclerosis (MS). The Phase 2 study of autologous MSC-NTF cells in patients with progressive MS (NCT03799718) started enrollment in March 2019. For more information, visit the company's website at www.brainstorm-cell.com
Safe-Harbor Statement
Statements in this announcement other than historical data and information, including statements regarding future clinical trial enrollment and data, constitute "forward-looking statements" and involve risks and uncertainties that could cause BrainStorm Cell Therapeutics Inc.'s actual results to differ materially from those stated or implied by such forward-looking statements. Terms and phrases such as "may", "should", "would", "could", "will", "expect", "likely", "believe", "plan", "estimate", "predict", "potential", and similar terms and phrases are intended to identify these forward-looking statements. The potential risks and uncertainties include, without limitation, BrainStorm’s need to raise additional capital, BrainStorm’s ability to continue as a going concern, regulatory approval of BrainStorm’s NurOwn treatment candidate, the success of BrainStorm’s product development programs and research, regulatory and personnel issues, development of a global market for our services, the ability to secure and maintain research institutions to conduct our clinical trials, the ability to generate significant revenue, the ability of BrainStorm’s NurOwn treatment candidate to achieve broad acceptance as a treatment option for ALS or other neurodegenerative diseases, BrainStorm’s ability to manufacture and commercialize the NurOwn treatment candidate, obtaining patents that provide meaningful protection, competition and market developments, BrainStorm’s ability to protect our intellectual property from infringement by third parties, heath reform legislation, demand for our services, currency exchange rates and product liability claims and litigation,; and other factors detailed in BrainStorm's annual report on Form 10-K and quarterly reports on Form 10-Q available at http://www.sec.gov. These factors should be considered carefully, and readers should not place undue reliance on BrainStorm's forward-looking statements. The forward-looking statements contained in this press release are based on the beliefs, expectations and opinions of management as of the date of this press release. We do not assume any obligation to update forward-looking statements to reflect actual results or assumptions if circumstances or management's beliefs, expectations or opinions should change, unless otherwise required by law. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements.
CONTACTS
Investor Relations:
Preetam Shah, MBA, PhD
Chief Financial Officer
BrainStorm Cell Therapeutics Inc.
Phone: + 1.862.397.1860
pshah@brainstorm-cell.com
Media:
Paul Tyahla
SmithSolve
Phone: + 1.973.713.3768
Paul.tyahla@smithsolve.com
Tools
Investors
- Overview
- News
- Events & Presentations
- Stock Information
- Financial Reports & Filings
- Corporate Governance